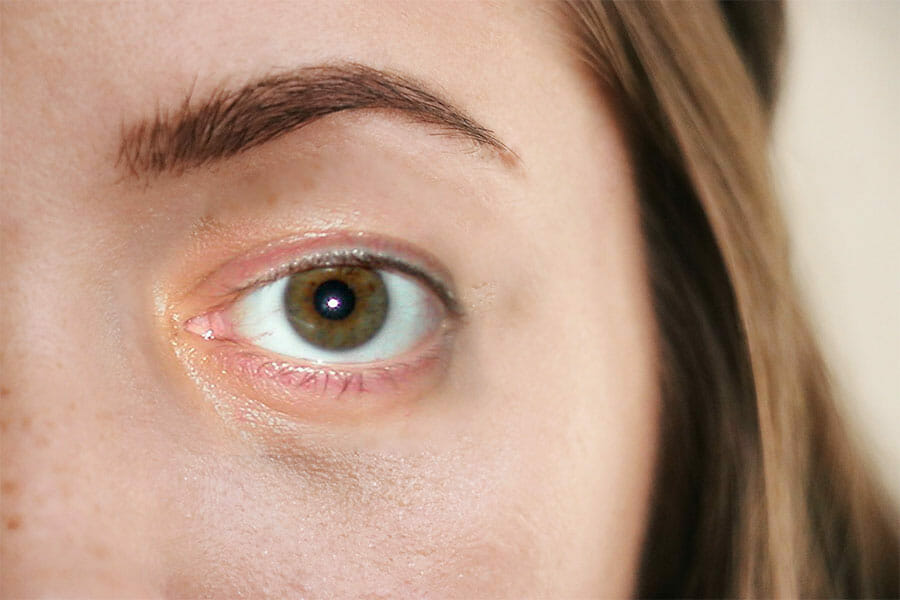
What conditions can amniotic membranes be used for?
Amniotic membrane is utilized in post surgical care after surgeries that involve removing eye tissues, including treating chemical burns, corneal ulcers and conjunctival ulcers, as well as diseases that cause painful ulcerations.
In a 12-year study conducted in Italy, 5,349 surgical procedures using amniotic membrane patches were successfully performed. The treatment's effectiveness was evaluated one year after surgery based on the scope of the surgery, resolution of inflammation, relief of symptoms, restoration of regular and stable corneal epithelium, and restoration of the structural integrity of the eye.
Consequently, the authors of the study concluded that the proposed procedure for the therapeutic use of amniotic membranes to treat various ocular pathologies is reproducible, and that amniotic membranes can be used in place of conventional medical treatment for certain conditions in the ocular system.
Conditions treated using amniotic membranes included:
- Neurotrophic keratitis
- Keratitis/endophthalmitis
- Pterygium
- Post-keratoplasty, glaucoma or cataract
- Chemical trauma
- Bullous keratopathy
- Neoplasm of the ocular surface
- Reconstruction of the conjunctiva and fornix
- Corneal degeneration
- Recurrent epithelial erosion
- Primary and secondary limbal stem cell deficiency
- Mucous membrane pemphigoid
- Dystrophy
- Mechanical trauma
- Chronic Stevens-Johnson syndrome or Lyell’s syndrome
- Reconstruction of the anophthalmic cavity
- Physical trauma
- Eyelid reconstruction
- Dysfunctional tear syndrome
In this study, partial success was achieved if two criteria were met. If any of these criteria were not met, the result was considered a failure. The study achieved a 100% success rate in treating a variety of conditions, including corneal ulcers and neurotrophic keratitis, as well as post-keratoplasty, glaucoma, cataract, bullous keratopathy, corneal degeneration and dystrophy, mechanical trauma, and reconstructing the anophthalmic cavity and eyelids. Additionally, dysfunctional tears were successfully treated in the study.